OCV supports FDA-compliant packaging line
Multiple machine-vision stations inspect and verify pharmaceutical vial-packaging system.
Amajor pharmaceutical manufacturer with a packaging facility in Puerto Rico recently asked Systech International to design, qualify, and install a vial-packaging line compliant with US FDA 21 CFR standards, including label application, tray filling, and palletizing. The line had to be flexible enough to accommodate new products or to add new sensors and software and to report capabilities without having to be recertified.
Any software or hardware change to a pharmaceutical manufacturing line must provide an audit trail for 100% of lot production. If a system—particularly software—is not properly designed and compartmentalized, changes to any part of the system can warrant a revalidation of the entire line. By using off-the-shelf modular software proven to comply with 21 CFR 211, only the new modules and affected supervisory functions must be validated when something is added to or subtracted from the production line.
The Systech manufacturing line includes two monochrome optical-character-verification (OCV) machine-vision inspection stations at the front-end cut-away labeler and back-end expiration-lot labeler, a third for Data Matrix verification, and a fourth color vision system to check color codes on the vials in packing trays before shrink wrapping, casing, and palletizing. Each vision station provides a key inspection and documentation step on the way to FDA compliance.
Single Point of Control
An end-to-end audit trial for pharmaceutical production and packaging is simplified if the line has a single point of operation and data I/O for all but operators with management clearance and above, according to Systech International regional sales manager Len Valeo. If the system has only one data I/O and control point, the likelihood of operator error altering a subsystem or resulting in lost data is greatly reduced.
Supervisory-control-and-data-acquisition systems for industrial manufacturing and automation inherently do not provide secure access or data tracking to 21 CFR levels. “About 75% of all pharmaceutical packaging equipment is not compliant with 21 CFR Part 11,” says Systech International regional sales manager Len Valeo. “Placing our Advisor Line Management Software on top of the Allen-Bradley PLC network enables the equipment with a form of compliance.”
A secondary proprietary physical layer based on a CAN network connects and secures all intelligent equipment on the line through a Systech TIPS LINK proprietary cable and Vision Integration Kit (VIK) I/O box attached to the PLC. The VIK provides a high-bandwidth data channel to the vision system, as well as access to the PLC through an embedded Allan-Bradley industrial Ethernet-compatible embedded chipset. The high-bandwidth channel allows for low-latency transmission of images from all three machine-vision inspection stations to the Systech central ISX computer, which runs Sentri vision software and associated image-processing modules along with the Advisor supervisory program and the main HMI for the pharmaceutical production line. The ISX is an industrial, Pentium-based PC with VIK master card to access the Systech network and Allan-Bradley SLC card to access the industrial Ethernet PLC network.
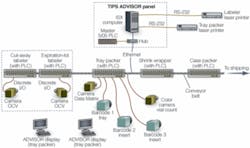
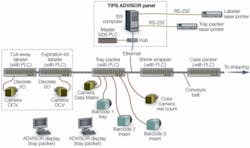
To initiate a lot run, a supervisory-level operator selects the production lot recipe from a library of recipes stored on the ISX central computer. The Advisor software queries the operator for each relevant variable (for example, lot size, expiration dates) and initiates the production run. Advisor sends out operational information to the cut-out labeler, expiration/lot labeler, tray-loading machine, smart barcode readers, and associated vision inspection stations, as well as resetting counters and relevant settings for each PLC on the Allan-Bradley network (see Fig. 1).
FIGURE 1. The Systech TIPS Advisor network for pharmaceutical production lies on top of the Allan-Bradley Ethernet network and uses minimal proprietary discreet runs, as well as the AB network, to communicate, initiate, secure, and control all equipment on the packaging line.
“The labeler and tray PLCs both have laser printers, while the lot/expiration code printer uses a Lastec laser etching system,” explains Valeo. “If Advisor wasn’t sending the information to these printers, the operator would have to manually input the information separately into each machine, increasing the chances of errors.”
Valeo says customers can save upward of $30,000 by eliminating the need for a complete barcode reader system and HMI and by using $5000 smart barcode readers from Microscan or Accu-Sort directly integrated to the Advisor program and HMI. Once the operator initiates the lot, Advisor sends a lock code to every intelligent machine on the system so that variables cannot be changed locally—with few exceptions.
Securing the Unsecured
The majority of packaging equipment used in pharmaceuticals is not 21 CFR compliant, which means the entire line is not FDA compliant. The cut-out labeler PLC, expiration/lot-code printer PLC, tray PLC, and shrink-wrap-palletizer PLCs are all connected to the Allen-Bradley industrial Ethernet network, but control is not secure against local access. Recipes for each machine’s operation are stored locally on the PLC, which does not include the security levels required to ensure production lot integrity based on 21 CFR standards. However, the PLC can be programmed for a variety of alarm codes.
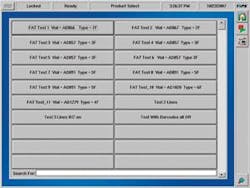
FIGURE 2. During 21 CFR testing to validate pharmaceutical production, the TIPs Advisor screen shows the operator a view of a factory acceptance test of vial-inspection packaging line.
Systech set aside a 1-bit alarm code based on data accessed at the PLC. If the local recipe is accessed, the alarm bit is set to its highest level of 3. The Systech system has full access to each PLC’s data via its connection to the Allan-Bradley industrial Ethernet LAN. When a Level 3 code is issued, Advisor halts the line. It can only be restarted by a management-level supervisor. So while the actual changes to the recipe cannot be guarded on the PLC, Advisor does track who approved restarting the line in response to a Level 3 PLC recipe access event, providing the requisite audit trail and making unsecured PLCs secure to 21 CFR levels (see Fig. 2).
Key Production Steps
After the operator initiates the operation and inputs all values and each PLC and vision system is programmed with the appropriate procedure based on the stored recipe in the Systech program, filled glass vials enter the labeler station from an accumulator. A laser printer inside the labeler machine begins printing the cut-out labels with the appropriate information, and an image of each label is captured by a Systech CCD-610 monochrome digital camera through a local trigger. The camera is outfitted with standard optics and bandpass filter to reduce interference from ambient light and focus on the illumination from a nearby Systech LED-RR01 red LED ringlight. The image is transferred back through the VIK box to the VIK master at the ISX host PC and then passed into the PC memory where the Systech OCV tool takes over.
If the label is passed by the Sentri inspection software, the labeler PLC extracts the label from the printed roll and attaches it to the vial. A second printer and monochrome vision system verify a Data Matrix code, which is then also applied to the vial. The vial passes through the labeler, and a smart barcode reader verifies a separate Code 128 dense barcode on the label against the programmed recipe. Using multiple codes provides redundancy in the operation.
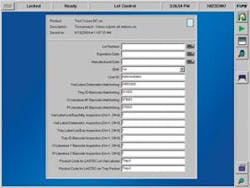
The vial then enters the lot-code/expiration-date laser printer, which etches the lot code into the glass in the cut-out area. A second OCV system verifies the lot code and expiration date (see Fig. 3).
FIGURE 3. Systech software for optical character verification allows the system to read slightly distorted or skewed codes.
The vial continues to the tray packer PLC. A pair of barcode readers read the Code 128 codes on the plastic tray and plastic insert and record those data with the lot information on the local PLC, which is then passed to Advisor. The PLC places vials in each of the tray’s holes and places a colored dot on the top of the vial that varies by recipe. When the tray is filled, a Systech CCD-645 megapixel color CCD camera captures an image of the entire tray from above and uses color thresholding to identify each colored dot. The dots are counted to verify the tray is full and checked against the color code included in the recipe.
Systech has a variety of color software modules for its Sentri vision systems for pharmaceutical production. One interesting feature is the ability to do 15-bit digital stacking of each color to identify all RGB color space values that are near to the target color code. The color tool creates a look-up table for each dot based on nearby color extrapolation. This allows the system to accommodate slight changes in the dot’s color without falsely rejecting the vial. Also, the tool generates a 3-D RGB color space cub that can show all colored labels used for lot tracking. This allows the engineer to verify that no colored dot can be mistaken for another dot to its proximity in color space.
After the color images are sent back via VIK channel to the ISX computer and passed, the Systech system sends a signal for the PLC to pass the filled tray to the shrink wrapper and robotic palletizer for shipping to the customer.
Features, advantages, benefits
“We use true optical character verification, which compares fonts to libraries of digital images,” explains Systech software engineer Mike Soborski. “We use digital stacking to create varieties of the code that include common skew and other distortions based on our knowledge of what typically happens in a pharmaceutical laser printer. Unlike items in the food industry, a vial of medicine may cost $100 and be produced at 350 vials a minute. Pharmaceutical manufacturers do not want this line to go down. Engineers spending their time tweaking thresholds or optical character recognition tools is simply not acceptable.”
null