Vision helps make blister packs safer
Combining a smart camera, PLC, and intelligent data terminal speeds pharmaceutical packaging inspection.
By David Lieberman, Contributing Editor
Millions of medicinal tablets and capsules are produced every year, and many of the over-the-counter varieties are encased in blister packs. While manual methods for inspecting these packages are subjective and lack repeatability, electronic techniques have been challenged by the great variation in blister packaging.
Perhaps the oldest technique for blister-pack inspection is the water-bath test. In this test, a sample package is immersed in water, a vacuum is applied, and the operator looks for bubbles and makes a judgment. Using this method, no statistical pass or fail result can be achieved. Other techniques such as dye penetration, in which the packs are immersed in dye, are unreliable and messy. Because both of these are destructive tests, repeat testing on flawed packages is not possible, and good product is discarded in the inspection process.
More sophisticated means of gauging, such as vacuum-leak testing, test the seal integrity of the blister pack. However, while this approach works well for testing the integrity of the pack as a whole, it cannot identify which chambers are flawed since it is not possible to isolate blisters individually in a vacuum. Of course, inspection techniques based on machine-vision systems can inspect the surfaces of blister packs; yet, since these systems are sensitive to differences in the surfaces they inspect, results will vary.
Combined methods
In both cases, vision alone is not sufficient to meet the quality-control requirements of pharmaceutical-packaging companies. To address this, Packaging Technologies & Inspection (PTI) has developed a system that combines vacuum-leak testing with machine vision, joining the strengths of both techniques in a single nondestructive-test machine. By combining vacuum-leak testing with a machine-vision system, leak testing reveals a flawed blister pack, and, once detected, a machine-vision system identifies the flawed blister chambers (see Fig. 1).
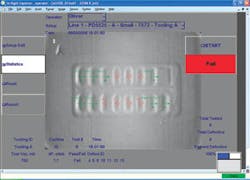
Figure 1. Vacuum-leak testing and visual inspection subsystems of the VeriPac 225/BLV are mounted in a drawer beneath the operator’s interface panel.
Logging into the system using a touch-sensitive LCD screen, the operator selects the identification code of the blister pack and enters the tablet type and manufacturer. The pack is inserted in a test fixture in the test chamber, and the chamber is sealed. The test fixture contains an embedded identification code, captured by an internal camera, that confirms the format and other characteristics of the blister-pack type.
The key to the integration of vacuum-leak testing and vision is a flexible structure. In operation, the system brings the flexible structure into contact with the blister-pack cover, gently compressing it. After a vacuum is applied, transducers measure the absolute and differential change in the vacuum in millibars per second to determine if air is seeping out from flawed chambers. The flexible structure supports the blister-pack cover to prevent the vacuum from adversely affecting blisters with good seals.
Vacuum testing causes faulty blisters to deflate, suffering a minute collapse compared to blister chambers without flaw. This creates a slight spatial displacement--a dip, imprint, or impression--in the flexible structure over blister chambers whose seals have not held (see Fig. 2). There is a discernable visual difference in appearance between good blister sites and sites that have been breached. The vision system then pinpoints which specific blister cavities are defective.
System design
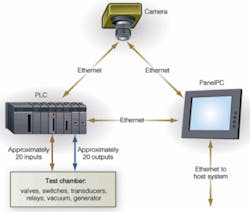
Three main electronic components comprise the VeriPac 225/BLV: an In-Sight 5400 digital camera from Cognex, a PLC from Direct Logic, and a PanelPC from Aaeon Technology (see Fig. 3). Packaged in a 1.62 × 4.91 × 2.43-in NEMA 6/IP67-rated aluminum housing, the Cognex In-Sight 5400 uses a 1/3-in. CCD to capture images in a monochrome 640 × 480 × 8-pixel VGA format. According to Stauffer, the VeriPac 225/BLV system does not place great demands on its imaging subsystem. Only a single captured image, for example, is required to determine the package quality and location of defects in a blister pack.
Figure 3. Ethernet is used to interface the system’s camera, PC, and PLC and allows the equipment to be interfaced to factory networks.
To control and monitor all the switches, valves, transducers, relays, and the vacuum generator, a Model DL06 from Direct Logic features a 229-item instruction set, and PTI puts it to work handling all the inputs to and outputs from the test chamber. In total, the VeriPac requires about 20 inputs and 20 outputs, according to Stauffer. The modular expansion capabilities built into the PLC allowed PTI to configure it for their interface needs. The PLC has two built-in serial communications ports and accommodates an Ethernet expansion module.
To run the custom front-end operator interface while providing the local storage for test results and images, a Model APC-8152 PanelPC from Aaeon Technology is used. This features a Pentium 4 or Celeron processor and up to 1 Gbyte of DDR DRAM. Test results and images are automatically stored on the APC-8152 2.5-in. IDE hard disk drive, along with statistical analyses of inspection trends. The PanelPC incorporates a 15-in. LCD with a 1024 × 768-pixel XGA format driven by a 4x AGP graphics card in the PC. An eight-wire resistive touch screen serves as the input medium of the operator interface.
Ethernet interfaces the PanelPC, PLC, and camera to each other and to the plant operator’s host computer system. The Ethernet port allows data to be exported to the host system and allows access to other nodes on the network. In addition to managing the operator interface, the PC in the PanelPC runs the custom image-analysis software that determines the integrity of the seal on individual blisters.
Features, advantages, benefits
According to Packaging Technologies & Inspection president Tony Stauffer, the VeriPac 225/BLV is the first system for blister-pack inspection that tests the integrity of the package and identifies leaks within individual cavities without destroying the product or package. Isolating seal problems to specific blister sites is critically important for the pharmaceutical-packaging industry, where statistical inspection with a high degree of repeatability provides a huge advantage.
“If you’re producing millions of blister packs and consistently have defective pockets in certain areas, you have a problem,” says Stauffer. “If you know where the problem is, then the problem in the packaging equipment can be rectified.”