Smart Cameras Track and Trace
Data management system employs smart cameras to validate pharmaceutical packaging
John DiPalo
To prevent drug counterfeiting, pharmaceutical oversight agencies are establishing stricter regulations for manufacturers, distributors, and retailers. The secure electronic version of the chain-of-custody for pharmaceuticals, often called ePedigree requirements, is soon expected to impact all pharmaceutical plants around the world.
Beyond compliance to ePedigree regulations, drug manufacturers see improved traceability as a way to minimize production and distribution of unsafe or poor quality products and reduce the potential for recalls.
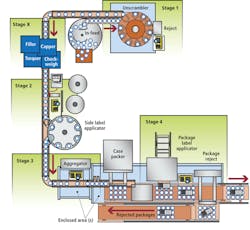
In response to these needs, Acsis has developed a serialized packaging data management (SPDM) system that uses smart cameras to validate the presence, accuracy, and readability ofpharmaceutical labels and ensure the integrity of packaged product (see Fig. 1). Comprised of six stages that can be implemented in total or independently of each other, the system allows pharmaceutical bottles to be identified and verified, and provides label printing, verification, bottle and label cross-referencing, and bundle, case, and pallet aggregation.
Identification and verification
Using an assigned, randomized identity, a unique two-dimensional (2-D) Data Matrix barcode is first printed directly on the container; this number is used as a container identifier and is typically unique for the batch. The container identifier is sent via an Ethernet network connection to the Acsis SPDM system and stored for future use in later stages of the process. This involves upgrading the integrated bottle unscrambling station from Omega Design Corp. to provide bottle inversion that enables underside printing with a Domino inkjet printer. The printing process is engineered in such a way as to not impact the speed of the line; it is fully integrated into the bottle unscrambling and has been tested at rates of more than 250 bottles per minute.
The unscrambler has been engineered to have all the requirements for inspection and rejection of unreadable bottles fully integrated within the footprint of the existing station. Then, aCognex In-Sight 5400 ID reader configured with In-Sight Explorer software is used to verify the accuracy and readability of the barcode (see Fig. 2). Missed or faulty printed units are immediately rejected into a secure container.
During the labeling process, an inline printer integrated into the labeler prints a GS1-compliant barcode that contains a unique serial number for the item as well as other variable data such as batch and expiration date onto the label. Typically this label is inspected on the web prior to applying it onto the bottle. If the label passes the verification, it is applied to the bottle; if not, either the label will be rejected prior to labeling a bottle or the labeled bottle will be rejected at a later point in the process. The preferred method is to have a labeler that can manage the reject process prior to labeling the bottle to minimize the amount of rework required in the event of a bad label. Then a series of processing stages such as insert/outsert verifications and seals are performed that, despite having no direct bearing on serialization, are critical to product safety and package integrity.
After the labels are printed and applied to the bottle, each bottle must be associated with a specific label. This involves associating the bottle/unit identifier to the label identifier. To achieve this, the bottle is presented to an array of two vision systems so the bottle/unit identifier and the label identifier are captured simultaneously and presented to the SPDM solution as a joined entity. Based on the joined data, the SPDM system verifies that the bottle/unit identifier and label data are valid. If there are inconsistencies, the system sends a reject signal and the product is rejected.
Aggegation stages
After containers are labeled, they must be combined into a bundle or a case. The 2-D barcode applied to the underside allows them to be identified after they have been aggregated into a bundle or case. The SPDM system then prints a label to the bundle or case label applicator to identify the aggregated unit. If bundling is used, the bundle is wrapped and a label applied to the top of it that includes a barcode identifying the bundle.
This method, using the identifier on the underside of the bottle, allows the system to provide a very robust method of aggregation since all of the bottles for a case layer or a bundle are read at one time. The alternative approach is to manage the state of all the items as they move into the bundler or case packer. This first in–first out approach can be used successfully if the material handling equipment can guarantee the order of the items throughout the process. The Acsis SPDM solution can manage either process.
At the end of the line, during case and pallet building, aFanuc robot lifts the items and presents them to a Cognex In-Sight 5605 vision system that captures images from up to 24 bottles (see Fig. 3). The vision system takes a single picture of the underside of the items and returns a full list of all units read on the image.
The SPDM system then reads and verifies each bottle/unit identifier against the system’s database, compares successful reads to the expected bundle size, and aggregates the associated label serial numbers to a unique bundle identifier. A barcode reader scans the individual barcode of each bundle. If all the expected values are identified, the SPDM system will allow the items to pass the bundle. If any of the expected units are not accounted for or if any of the barcodes are missing or illegible, the vision system will return a fail and the robot will place the bundle on the reject conveyor.
Once the items have been placed in a case, the solution will create a serialized case label for application to the case. This label can be applied automatically on the line or manually if a manual case packing process is being used.
Pallet aggregation is performed in a similar manner. First, the operator scans each case identifier; when the pallet is completed, the solution sends a pallet label print job to a label printer. Again, a barcode reader scans the label and the SPDM system returns an error/reject message if an unrecognized bundle identifier is read or if the identifier is not correctly associated to the packaging order.
Similar to the labeling tasks, the case and pallet building tasks have been optimized to ensure that the addition of serialization has a minimal impact on the speed of the line.
As an extension to Acsis’s ProductTrak for Life Sciences, the Acsis SPDM system is currently installed at several major pharmaceutical companies, allowing them to incorporate data serialization into new and existing packaging lines while maintaining existing machine speed and quality.
John DiPalo is chief technology officer at Acsis (Marlton, NJ, USA).
Company Info
Fanuc Robotics
Rochester Hills, MI, USA
Omega Design Corp.
Exton, PA, USA
More Vision Systems Issue Articles
Vision Systems Articles Archives