Pharmaceutical vendors to adopt Braille standard
Andrew Wilson, Editor, [email protected]
In 1825, Louis Braille invented a system used worldwide for the visually impaired for reading and writing, known as Braille. Originally developed to allow soldiers to pass secret information between each other in the battlefield, the Braille system is currently being adopted by major European pharmaceutical packaging companies.
According to the European Commission 2001/83/EC, all new pharmaceutical packaging must already be embossed with Braille, and all current packaging must support the Braille system by November 2010. To expedite this process, the specialist packaging alliance Copapharm Europe (Brussels, Belgium; www.copapharm.de) established a Braille standard that uses an embossing procedure integrated within packaging manufacturing systems.
Braille consists of arrangements of dots that make up the letters of the alphabet, numbers, and punctuation marks. To emboss a specific character or number, packaging manufacturers use the Braille cell, a rectangular grid that consists of six dots in two parallel vertical lines of three dots each. Braille alphabet characters are created from this basic Braille grid. Thus, a Braille character consists of six dots, positioned like the figure six on a die. From the six dots that make up the basic grid, 64 different signs can be created to form the basic Braille alphabet.
“From the outset, members of Copapharm realized the need to standardize the method for embossing Braille characters on pharmaceutical products,” says David Anderson of the University of Oklahoma (Tulsa, OK, USA; www.ou.edu). “Thus, set procedures were developed for a system that specifies the font type and size, forming and positioning of Braille characters, as well as methods that standardize the pharmaceutical cartons used and how the characters once formed could be properly tested.”
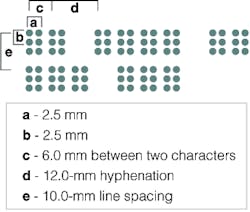
Marburg Medium is the most commonly used font to emboss Braille (see Fig. 1). In specifying this font, the European Commission For Use on Pharmaceutical Packaging and Labels and the European Carton Makers Association (The Hague, The Netherlands; www.ecma.org) have adopted this specification for their ECMA Euro Braille Standard. Specifying dot diameter, dot spacing, character and line spacing, and the dimensions of artwork spacing makes it easier for developers of automated machine-vision systems to develop optical character readers to automatically interpret the dots.
Figure 1. Marburg Medium is the most common font used to emboss Braille. Specifying dot diameter, dot spacing, character and line spacing, and the dimensions of artwork spacing makes it easier to develop optical character readers to interpret the dots.
With several clients in the pharmaceutical industry required to comply with the changes in packaging law, on-line companies such as Pharmabraille (www.pharmabraille.co.uk) have developed a range of fonts that can be readily placed on packaging or artwork. The company’s PharmaBraille range of Braille fonts, for example, follow the recommended Marburg Medium specification and the Euro Braille Standard of the European Computer Manufacturers Association (ECMA; Geneva; Switzerland; www.ecma-international.org).
“Knowing the size and location of the Braille dots, optical-character-recognition (OCR) systems can be developed to read and interpret the code placed on pharmaceutical packages,” says Anderson. “To read these embossed characters, however, requires the proper lighting techniques.” While on-axis illumination can be used to read the printed characters on the pharmaceutical label, darkfield lighting must be used to highlight the Braille characters. “In darkfield lighting, the light does not fall directly into the objective and the illuminated area appears dark, while defects (which are, in this case, the Braille characters) appear bright.”
At NIWeek 2007, Anderson demonstrated an OCR reader capable of interpreting Braille fonts using the latest smart camera (NI 1742) from National Instruments (Austin, TX, USA; www.ni.com). Based on a 1/3-in. CCD from Sony (Park Ridge, NJ, USA; www.sony.com/videocameras), the 60-frame/s camera incorporates a 533-MHz PowerPC, Gigabit Ethernet interface, four optoisolated I/O ports, and a built-in LED lighting strobe controller.
“Because the camera features direct-drive lighting control,” says Anderson, “it was possible to power and control the illumination of a 5-in. LED off-axis ringlight from CCS America (Burlington, MA, USA; www.ccs-inc.co.jp) directly from the camera.” This off-axis lighting was used to properly illuminate Braille characters that were embossed into a Tylenol package by hand with a Braille slate (see Fig. 2).
Figure 2. To read Braille characters, the known ROIs containing individual dots were first isolated using LabVIEW running directly on the camera. These isolated regions were then interpreted with the context of the Braille cell to automatically read the information contained within the package.
To read the Braille characters, the known regions of interest containing individual dots, as specified by Copapharm Europe, were first isolated using LabVIEW running directly on the camera. These isolated regions were interpreted with the context of the Braille cell to automatically read the information contained within the package. “Writing the OCR software in LabVIEW allows the software to be implemented in a number of different platforms including NI’s family of smart cameras, the company’s Compact Vision System, or on a standard PC host,” says Anderson. To make the code readily available to other developers, Anderson has placed the LabVIEW code at www.ni.com/vision.