OCR/OCV vision systems improve reject rates and quality yield on pharmaceutical line
A pharmaceutical label-selection application requires OCR/OCV and barcode reading to reduce the number of falsely rejected packages
A pharmaceutical manufacturer’s packaging line poses a challenge for vision systems because of the need to select the right label among many alternatives needed to meet differing languages and regulatory requirements around the world. The vision system used in the past by the manufacturer rejected 25% of good packages.
The packaging line used previously in the application was a linear machine with hot stamp pressure-sensitive printing and an image-comparison vision system. The vision system had difficulty matching ideal to actual images, which led to a high false reject rate. This high false reject rate played a major role in the line’s overall equipment effectiveness (OEE; see definition below text). The vision system also took a considerable period of time to set up for new labels.
The pharmaceutical manufacturer began looking at alternatives for improving the line. The company developed a rotary labeling machine using thermal transfer printing that offers higher availability and performance. For help in improving the vision system, the company began working with Wilfredo Jiménez and Jesus Otero from WJ Automation & Integration Corp. (WJAI; San Juan, Puerto Rico).
Selecting the vision systems
When the pharmaceutical manufacturer installed a new packaging line, the company implemented In-Sight 5100 and 5600 vision systems from Cognex (Natick, MA, USA), which use optical character recognition/optical character verification (OCR/OCV) to read characters on the label and compare them to the correct sequence. This approach reduced the false reject rate to 0.5%, making a substantial contribution, improving the first pass yield (quality) and consequently the OEE by 200%.
"When I first got involved in the project, the company was working with a machine builder to design two rotary label machines," Jiménez says. "They needed to inspect every aspect of a very complex label with near 100% accuracy." The initial challenge was in determining what vision system could accurately identify and verify many different labels. "We picked Cognex In-Sight 5100 and 5600 vision systems because they are fast, accurate, easy to program, and compact enough to fit easily within the available envelope of the machine," he explains.
"One of the problems with the previous system was that it used a separate camera, frame grabber, and computer, which were connected together by cables," Jiménez continues. "The interconnections generated a lot of noise, which was one reason for the poor accuracy of the vision system. The In-Sight [systems] avoid this problem because the complete vision system is self-contained within a single housing. Both vision systems are very fast, providing a 100-ms inspection cycle, which is well under the cycle time of the machine." The In-Sight 5100 vision systems acquire up to sixty 640 x 480-pixel full frames/s with 8-bit images. The In-Sight 5600 acquires 640 x 480-pixel images at very high speeds.
Programming the systems
Depending on the specific label that is used, information can appear on the right side, left side, or on both sides of the label. The left side of the label normally has a maximum of 3 lines of text with a maximum of 15 characters on each line. The right side of the label normally contains the Pharmacode, also known as Pharmaceutical Binary Code, a 1-D barcode standard used in the pharmaceutical industry as a packing control system. Some labels also have 2-D barcodes that could be on either side of the label. A 1-D barcode that defines the lot also appears across the bottom of each label. This means that a relatively larger field of view (FOV) of 4 in. wide and 2.5 in. tall must be read to fully verify the label. The glossy labels have a tendency to create reflections that can detract from image quality. Another inspection required was to inspect that the bottom side of bottles are properly printed.
WJAI divided the FOV into left, right, and bottle bottom sections, and addressed each section with a separate vision system. They used a Cognex In-Sight 5600 for the left side, an In-Sight 5100 for the right, and another In-Sight 5100 for bottle bottom side. The left vision system has an FOV of 1 x 2 in., the right system has an FOV of 1.5 x 2 in., and the bottom system has an FOV of 2 x 2 in. The design team used a diffused light and experimented with the lighting angle to minimize reflections.
WJAI used Cognex In-Sight Explorer software to program the cameras to read the labels. On the left hand camera, Cognex OCR/OCV tools were used to read the three lines of text and compare them to the expected result. Cognex IDMax code reading tools read the barcodes. The machine production rate could be tripled from its current 100 bottles per minute without increasing the vision system speed.
Integrating the vision systems with the packaging line
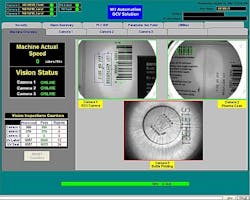
WJAI configured the vision systems to communicate with the Allen-Bradley programmable logic controller (PLC) and human machine interface (HMI) software that run the machine. When the operator begins running a new batch of labels, he or she first selects the type of label in the HMI which, in turn, informs the vision system of the text and barcodes that should be on the label and their position. This integration eliminates the need for setting up the vision system when changing from one type of label to another. The PLC also sends a signal that the label is in position, ready to be inspected. After inspecting the label, the vision systems provide the results to the PLC and HMI. Bottles that fail the inspection automatically go into a bad parts bin for manual inspection and repair. The operator can see images from the camera in real time on the control window, which makes it easy to resolve any issues.
The new vision system took only one week to validate, compared to three months for the previous system, reports Jiménez. It exceeded the pharmaceutical manufacturer’s expectations by providing a false reject rate of only 0.5%. It also helped to improve the performance (standard versus actual) of the packaging line by smoothing the progress of changing over from one type of label to the next, consequently improving the manufacturer's OEE.
| Overall equipment effectiveness |
| The overall equipment effectiveness (OEE) is the ratio of the fully productive time to the planned production time and can be calculated as a product of its three contributing factors: OEE = Availability x Performance x Quality. This type of calculation makes the OEE a severe test. For example, if all three contributing factors are 90.0%, the OEE would be only 72.9%. The generally accepted world class goals for the three factors are: Availability – 90.0%, Performance – 95.0% and Quality – 99.9%, which yields an OEE of 85.0%. Worldwide studies indicate that the average OEE rate in manufacturing plants today is around 60%. |
SOURCE: Cognex
-- Posted by Vision Systems Design