Researchers Develop Experimental Dual-Modality 3D Imaging System
Researchers at the Indiana University School of Optometry (Bloomington, IN, USA; https://optometry.iu.edu/) developed a multimodal imaging system combining structural and functional imaging to enable 3D visual evaluation of cell-level activities involved in developmental or disease conditions of the eyes.
Specifically, the researchers used high-resolution optical coherence microscopy (HR-OCM), a 3D structural technique, and dual-channel scanning confocal fluorescence microscopy (DC-SCFM), a functional technique, to simultaneously register imaging information. The researchers say the combined system could lead to imaging processes that allow biologists to evaluate changes in cellular activity in the eyes of small animals over time. For example, scientists could tag and then follow a specific protein within a cell using the functional system and the area around the cell, such as neighboring cells, with the structural system.
“We want to be able to capture not only the functional aspect of activities of the cells but also capture the structure around it, so we can have a full understanding of how the cells behave in their natural environment,” explains Patrice Tankam, an assistant professor at the Indiana University School of Optometry.
It is also important to follow the same animals. If scientists follow different animals or tissue samples, they do not know if differences they see are the result of biological changes or inherent variation in the subject matter, Tankam adds. Overall, he says, “Our goal is: How can we follow these biological processes on the same animals over time?”
This type of investigative work not only improves scientists’ understanding of the biology of the eyes but also could become an early step in developing new medications for people. “It will help scientists find a cure for certain diseases,” explains Reddikumar Maddipatla, a research associate at the Indiana University School of Optometry.
The researchers chose to coregister functional and structural imaging information because this would reduce the potential for misinformation more effectively than a sequential approach where you “capture the structural information and then you move the mice to a different system and collect the functional information,” Tankam says.
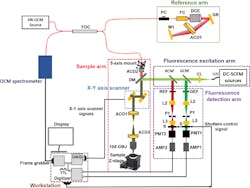
To create the dual-modality imaging system, the researchers overcame technical challenges so that both the HR-OCM and DC-SCFM systems record visual information from the same location at the same time. They wanted to maximize both lateral and depth image resolution.
Since the two systems operate in different parts of the light spectrum—OCM uses near infrared light and fluorescence uses visible light—they tend to focus on two different planes, which is known as focal plane mismatch, explains Maddipatla.
In addition to issues with focal plane mismatch, the two systems also record depth information differently, Maddipatla says.
The researchers addressed these problems in the design of the dual-modality system, which includes an HR-OCM reference arm and both a DC-SCFM fluorescence detection arm and excitation arm. They defocused the light beam from the HR-OCM system to align the systems along the plane of the sample.
To combine light signals by the two systems, Tankam and Maddipatla used Semrock’s (Rochester, NY, USA; www.semrock.com) dichroic mirror. From there, the signal went to a dual-axis galvanometric scanner from Cambridge Technology, a brand owned by Novanta Photonics, (Stockport, UK; www.novantaphotonics.com), which then relayed the light on to the sample.
In the HR-OCM system, light, which is transmitted through the mirror, is then detected by a spectrometer from Wasatch Photonics (Morrisville, NC, USA; https://wasatchphotonics.com). The spectrometer is equipped with a Teledyne e2v OctoPlus (Milpitas, CA, USA; imaging.teledyne-e2v.com) high-speed line scan camera operating at 250 kHz.
The electrical output signal from the spectrometer is then recorded by an Axion CameraLink frame grabber from Bitflow (Woburn, MA, USA; www.bitflow.com.)
In the second imaging system, the fluorescence emission signal from the fluorophores travels from the sample back to the mirror where it is reflected to the fluorescence system through two channels—red and green. The light is then focused by an aspheric lens from Thorlabs and transmitted through Thorlabs’ (Newton, NJ, USA; www.thorlabs.com) 10-µm pinhole aperture – P1, a product that only lets through rays coming from a single plane inside the sample while blocking light rays from other planes or depths. Next, another lens, also from Thorlabs, adjusts the beam size before funneling it through a photo-multiplier tube from Hamamatsu (Hamamatsu, Japan; www.hamamatsu.com). The output from the tube is converted to voltage using FEMTO amplifiers (Berlin, Germany; www.femto.de). A four-channel digitizer from Alazar Technologies (Pointe-Claire, Canada; www.alazartech.com) is used to record the voltage from the amplifiers.
For the last step, the researchers recorded and synchronized the HR-OCM and DC-SCFM image data using custom software they developed using National Instruments’ (Austin, TX, USA; www.ni.com) LabVIEW 2017. They used both LabVIEW and MATLAB from MathWorks (Natick, MA, USA; www.mathworks.com) for further image data processing.
To test the system, they used custom-made phantoms representing mice, which were constructed using imaging fluorescence microspheres embedded in multilayer tape and silicone.
The combined system reached a speed of 250 kHz and a lateral resolution of 2 µm, and an axial resolution of 2.4-μm was captured over a field of view of 1.1 mm × 1.1 mm.
For the next phase, Tankam and Maddipatla plan to test the system on tissue samples before tackling their ultimate goal of following cellular activity in the eyes of small animals.
About the Author
Linda Wilson
Editor in Chief
Linda Wilson joined the team at Vision Systems Design in 2022. She has more than 25 years of experience in B2B publishing and has written for numerous publications, including Modern Healthcare, InformationWeek, Computerworld, Health Data Management, and many others. Before joining VSD, she was the senior editor at Medical Laboratory Observer, a sister publication to VSD.