Revealing Eye
Microscopy system based on 3-CCD color camera, frame grabber, and off-the-shelf software enables researchers to image mouse retinas
Winn Hardin, Contributing Editor
Researchers derive important biomedical data by imaging the retina of mice. To provide these researchers with a variety of imaging modalities and wide-field, high-resolution, in vivo capabilities, a new retinal imaging system has been developed.
Biomedical research based on retinal imaging has become the starting point for testing new drugs and for assessing early toxicity in drug studies. Mice are especially attractive for genetic research because they have a particularly plastic genome. For example, young mice can be engineered to have various diseases that mimic human dystrophies such as diabetes and Alzheimer’s disease.
Although mice are useful for this research, imaging the retina of a mouse’s 3-mm diameter eye with sufficient spatial resolution is challenging. Attempts at using cameras designed for the human eye such as the scanning laser ophthalmoscope (SLO) or the classical fundus camera have produced limited results and at great expense.
As a result, researchers have been forced to breed large colonies of animals, sacrifice a segment of the colony at each stage of disease progress, remove the retina, and flat-mount it to a microscope slide for examination. A camera that can image in vivo would allow important longitudinal studies.
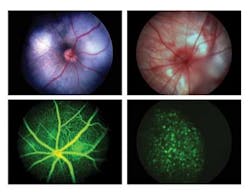
In addition to the basic requirement for wide-field, high-resolution in vivo imaging, researchers such as Knut Stieger, DVM, PhD, Department of Ophthalmology, Justut-Liebig-University in Gießen, Germany, have stated a need for a retinal microscope that would operate in multiple imaging modalities. These included white light or “brightfield” imaging, angiography to study the retinal blood flow by injecting the dye fluorescein, and imaging fluorescent molecular probes such as green fluorescent protein (GFP), along with other fluorophores.
While some results have been obtained, the vast difference of the eye design and size as compared to the human create a significant barrier for the design of retinal cameras. The SLO faces similar challenges and in addition cannot provide arbitrary wavelengths for fluorescent observations.
In response to these requirements for a multiple modality, in vivo retinal imaging microscope, Phoenix Research Laboratories developed the Micron III retinal-imaging microscope. The Micron III integrates a custom optical system with a xenon light source and Toshiba Imaging IK-TF7 three-chip CCD camera with a Euresys Domino Harmony frame grabber in a PC running NorPix StreamPix image-recording software (see Fig. 1).
Miniscule motion
The optical design of the mouse eye is considerably different from that of the human eye as well as being 1/8 the diameter. This necessitated custom optics, especially for the stressing requirement for a wide field of view and high resolution. Just as challenging to design was the means for injecting sufficient light into the tiny eye while avoiding glare from the optics and scatter from the eye.
A significant consequence of the 4-μm resolution is a shallow depth of focus of approximately 20 μm. The heartbeat and respiration of a mouse cause it to move more than 20 μm, which meant the system needed to accommodate these motions to obtain the best images.
Bert Massie, PhD, president of Phoenix Research Laboratories, concluded that image capture had to provide full resolution at all colors—for the various fluorophores—and also be in real time with full digital resolution to capture the images that are in focus as the animal moves over 20 μm. These requirements drove the component selection, Massie says, especially the digital video requirement that dictated the capture of 30 frames/s at XGA resolution with the 24-bit digital depth of a still image.
To deliver high light levels across the visible selection for the fainter fluorophores, Massie chose the Perkin Elmer 300-W Cermax xenon lamp. The source has a high brightness (meaning watts per steradian per square meter and over the entire visible spectrum). This light was filtered to allow only wavelengths between 400 and 700 nm, and then injected into a high-temperature fiber provided by Fiberguide Industries. The optical system also includes Semrock excitation and barrier filters. Both the excitation and barrier filters are mounted on thumbwheels, allowing the filters to be readily switched for various fluorophores (see Fig. 2).
“The fundamental requirement for imaging not only in color but at various fluorescent wavelengths leads to the requirement for high spatial resolution across the entire visible band,” explains Massie. “A standard single-chip CCD camera cannot deliver the required performance because it utilizes a Bayer filter mask over the pixels with an array of color filters to capture a color image. This reduces the spatial resolution and color specificity of the sensor, which is important for fluorescence imaging.
“The Bayer filter provides twice the resolution in green as red or blue,” Massie adds. “Software interpolates the colors in between where measurements are not made. In contrast, the three-chip CCD uses a prism to separate the colors and each color is then sent to one of three CCDs. This allows equal spatial resolutions for each color, which is critical for fluorescent studies at arbitrary wavelengths.”
Massie notes that the Toshiba Imaging IK-TF7 three-chip CCD camera was selected for its high resolution (XGA), small size (56 × 44 × 44 mm), full pixel-independent readout of 30 frames/s, and high dynamic range of 1000:1. The three 1/3-in. progressive-scan CCDs eliminate image jitter caused by combining two interlaced image fields, which is also important to live animal retinal imaging tasks.
To overcome the movements of the mouse during imaging, Massie designed the system to operate at 30 frames/s. The images are captured by a PC containing a Euresys Domino Harmony frame grabber, selected because it can perform 24-bit RGB capture at 30 frames/s.
The digital images are passed from the frame grabber to the StreamPix software, which Massie chose as a cost-effective solution for image acquisition and management. This combination of boards, camera, and software leads to the ability to record data from the mouse in vivo, and then, during post-imaging, extract the best frames into a common format such as BMP or TIFF.
Massie adds, “A decision was made to not add image-processing or evaluation functions to the system computer. There are quite a few codes such as Photoshop or MATLAB that specialize in this. The data are captured in a linear or ‘diagnostic’ mode to allow science to be done. Most cameras, including most retinal cameras, apply a nonlinear transfer function to the data to improve contrast. For scientific analysis, a linear function is needed. Accordingly, for presentation and improved visualization when the image may be affected by haze, most good image-processing codes can be used for contrast enhancement.”
The Micron III microscope is currently used by leading drug companies and universities in Europe, Asia, and the United States for a variety of imaging modalities (see Fig. 3). “Exceptionally good imaging system for rodents,” explains Justut-Liebig-University’s Steiger. “The whole system is very user friendly, which makes it possible to finish the complete examination of an animal before any cataract can occur. Especially the angiography function produces high-quality images, where you can see single cells moving within the vessels. The use of this system to image mice with pigmented retinas is also absolutely possible.”
“I have always sought good fundus images,” says Robert L. Peiffer, Jr., DVM, PhD, senior investigator with Merck & Co. “It used to take all day for an experienced researcher to get just one. Now with our Micron, even a new user can learn to capture publishable rat and mouse fundus images in less than 30 minutes.”
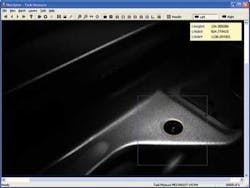
Massie has added several new capabilities to the Micron III to create a comprehensive eye research system. These modalities, attaching at the objective lens, image the front of the eye, for example (see Fig. 4).
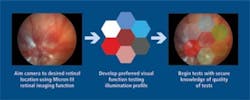
Another attachment allows the researcher to image the retina to verify alignment and then project into the eye an arbitrary pattern of light (see Fig. 5). The eye’s electrical response is then measured and researchers can determine the patency of the eye’s electrical system and as a function of location in the retina. Plans are underway to extend the basic design to larger animals such as rabbits and monkeys.
FIGURE 5. For visual function testing, an attachment allows the camera to image the retina to verify alignment then project an arbitrary pattern on the retina and measure the electrical response.
Company Info
Euresys
Angleur, Belgium
www.euresys.com
Fiberguide Industries
Stirling, NJ, USA
www.fiberguide.com
NorPix
Montreal, QC, Canada
www.norpix.com
PerkinElmer
Waltham, MA, USA
ww.perkinelmer.com
Phoenix Research Laboratories
San Ramon, CA, USA
www.phoenixreslabs.com
Semrock
Rochester, NY, USA
www.semrock.com
Toshiba Imaging Systems Division
Irvine, CA, USA
www.cameras.toshiba.com